Extra, extra! Read all about it!
MDEA announces its 2014 medical technology winners!
By Rick Fromme
 If you’re a medical device sales professional, or you’re
looking into transitioning into this exciting field of health care, then this blog’s
for you. In our ongoing series of highlighting advanced medical technologies across multiple disciplines (i.e., imaging, diagnostics, surgical, consumer,
etc.), we strive to introduce to some of the most innovative and life-changing
products being produced world-wide today.
If you’re a medical device sales professional, or you’re
looking into transitioning into this exciting field of health care, then this blog’s
for you. In our ongoing series of highlighting advanced medical technologies across multiple disciplines (i.e., imaging, diagnostics, surgical, consumer,
etc.), we strive to introduce to some of the most innovative and life-changing
products being produced world-wide today.Breaking News
You may recall our second edition of “FascinatingCaptain!” featured the award-winners of the 2013 Medical Device and
Engineering Awards (MDEA). As stated by “MD+DIMagazine” about its prestigious award ceremony:
“The Medical Design Excellence Awards (MDEA) competition is
the medtech industry's premier awards program recognizing the highest caliber
medical devices and diagnostic products on the market. The MDEA program
recognizes the achievements of medical device manufacturers, their suppliers,
and the many people behind the scenes who are responsible for the cutting-edge
innovations that are saving lives, improving patient healthcare, and
transforming medtech worldwide — one innovation at a time. Since its
inception in 1998, the competition has honored over 600 groundbreaking
products.”
Earlier this week, during its annual MDEA Conference and
Exposition in New York City, “MD+DI Magazine” announced its international award
winners for the most compelling and innovative medical devices for 2014. Below
are the Gold winners in each of the award ceremony’s 11 distinct categories:
Critical Care & Emergency Medical Products
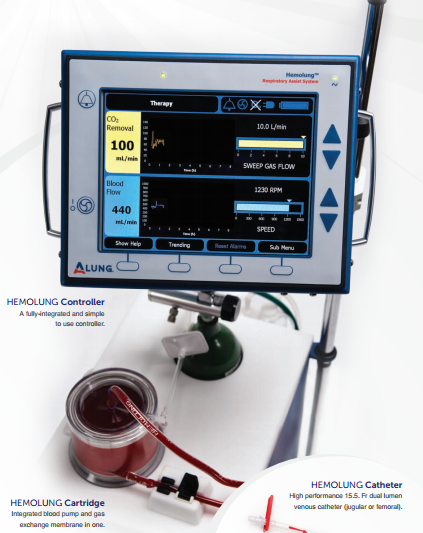
Hemolung RAS extracorporeal carbon dioxide removal
system. Manufactured and submitted by ALungTechnologies Inc. (Pittsburgh). Hemolung RAS provides respiratory dialysis;
a simple, minimally-invasive form of extracorporeal carbon dioxide removal
(ECCO₂R) that acts as an alternative or supplement to mechanical ventilation in
cases of acute respiratory failure.
Dental Instruments, Equipment & Supplies
Solea CO₂ dental laser system
for hard and soft tissue. Manufactured by Convergent Dental Inc. (Natick, MA). At 9.3 μm, Solea is the first CO₂
laser system ever cleared by the FDA for hard and soft tissue ablation. Solea
is fast, precise, virtually noiseless, and anesthesia-free for the vast
majority of procedures.
Drug-Delivery Devices and Combination Products
 PROPEL mometasone furoate implant and PROPEL mini
mometasone furoate implant. Manufactured and submitted by
PROPEL mometasone furoate implant and PROPEL mini
mometasone furoate implant. Manufactured and submitted by
General Hospital Devices & Therapeutic Products
LuViva Advanced Cervical Scan is a point-of-care, noninvasive cervical disease detection device. Manufactured by Guided Therapeutics Inc. (Norcross, GA). LuViva is a test for early detection of the disease that leads to cervical cancer. It does not require a tissue sample. http://www.guidedinc.com/
Implant & Tissue-Replacement Products
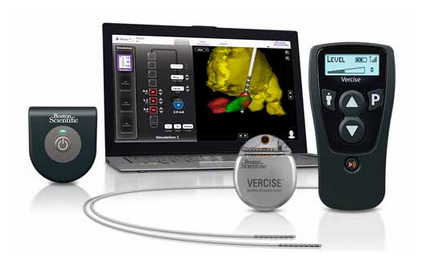 GUIDE DBS programming planning and visualization system
for deep brain stimulation (DBS). Manufactured and submitted by Boston Scientific Corp.
(Natick, MA). GUIDE DBS is a software program that creates a 3D image of a
patient’s deep brain anatomy and the relative location of the implanted lead. A
model of the volume of activated tissue allows the clinician to intelligently
optimize stimulation parameters.
GUIDE DBS programming planning and visualization system
for deep brain stimulation (DBS). Manufactured and submitted by Boston Scientific Corp.
(Natick, MA). GUIDE DBS is a software program that creates a 3D image of a
patient’s deep brain anatomy and the relative location of the implanted lead. A
model of the volume of activated tissue allows the clinician to intelligently
optimize stimulation parameters.
Invitro Diagnostic Products & Systems
AtomoRapid HIV (1&2) integrated rapid antibody test. Manufactured
and submitted by Atomo Diagnostics PtyLtd. (Sydney, Australia). AtomoRapid HIV is the first integrated rapid test
for detection of HIV 1&2 using blood. The AtomoRapid blood testing platform
can accommodate test strips for a wide variety of condition such as celiac
disease, allergies, and infectious diseases such as malaria and HIV. The device
delivers better clinical outcomes for patients and health care providers by
enabling simpler, safer and more accurate testing in a field or at home.
Medical Product Packaging, Graphic Instructions &
Labeling Systems
SoTube/SoSafe double-sterile barrier packaging.
Manufactured and submitted by SeleniumMedical (La Rochelle, France). SoTube/SoSafe are two innovative solutions—
nested tubes based for implants in a double-sterile barrier packaging. This
ensures nurses and surgeons easy handling of package and implants, as well as a
pioneer “No Touch” approach right up to the point of surgery.
Over-the-Counter & Self-Care Products
AEROBIKA oscillating positive expiratory pressure
therapy system. Manufactured and submitted by Trudell Medical International (London,
Canada). The AEROBIKA oscillating PEP device helps loosen and remove mucus
build-up in the lungs.
Radiological & Electromechanical Devices
AIRO Mobile Computed Tomography (CT) System.
Manufactured and submitted by MobiusImaging LLC (Ayer, MA). The AIRO Mobile CT integrates advanced imaging
technologies into existing medical workflow and provides the clinician with
procedural flexibility as well as real-time CT imaging information where and
when it’s needed.
Vector Gait and Safety System. Manufactured and submitted by
Bioness Inc. (Valencia, CA). The Vector Gait and Safety System is a ceiling-mounted robotic technology designed
to improve patient mobility and functional independence by tracking movement
and offloading a precise amount of body weight during rehabilitation.
Surgical Equipment, Instruments & Supplies
 Zip Surgical Skin Closure. Manufactured and submitted
by ZipLine Medical Inc. (Campbell,
CA). Zip is a noninvasive skin closure alternative to sutures and staples for
surgery and lacerations, offering time-savings, better cosmesis, lower
infection risk, and greater patient comfort. It’s easy to apply and patients
can remove it at home.
Zip Surgical Skin Closure. Manufactured and submitted
by ZipLine Medical Inc. (Campbell,
CA). Zip is a noninvasive skin closure alternative to sutures and staples for
surgery and lacerations, offering time-savings, better cosmesis, lower
infection risk, and greater patient comfort. It’s easy to apply and patients
can remove it at home. Of those Gold winners cited above, Atomo Diagnostics’ AtomoRapid HIV was chosen as this year’s overall “Best in Show.”
AtomoRapid
 MDEA’s “Lifetime Achievement Award” winner was Dean
Kamen, the inventor of the new, FDA-approved DEKA prosthetic arm.
MDEA’s “Lifetime Achievement Award” winner was Dean
Kamen, the inventor of the new, FDA-approved DEKA prosthetic arm.
It took eight years of research, but Kamen, the inventor of
the Segway “scooter,” created the closest thing to a replacement for
amputated arms — and industry pundits acknowledge it’s a game changer. It
allows users to zip up coats, gently handle eggs and drink from a bottle of
water, among other tasks.
Developed by Kamen’s DEKA Research and Development,
along with funding from the DefenseAdvanced Research Projects Agency (DARPA), the device can be fitted for
people with amputations at the shoulder, upper arm or lower arm, but not at the
elbow or wrist. (For more information on some of the cool projects DARPA funds
or sponsors, read our blog, “TheNext Generation — Putting Your Best Robot Forward.”)
Nicknamed “Luke” after the “Star Wars” hero who lost his
hand in a light-saber duel, Kamen’s device works by picking up on the
electric signals near the point of amputation and translating those to the
prosthetic. It’s a transparent replica
of a human arm and hand, complete with fingers and a thumb.
“Luke” gives users
the ability to perform multiple functions at one time, an advance over most
available prosthetics.The human brain automatically calibrates the amount of grip
needed to pick up objects, and knows to adjust strength in order to handle a
coin as opposed to a book. The “Luke” arm does the same, switching between six
different grips as the wearer decides. Being able to control several joints at
the same time also increases users’ range of motion, allowing them to open a
lock with a key, or chop food to prepare a meal.
In this article, I shared with you the hot-off-the-presses
Gold award and special category winners of this year’s MDEA medtech awards,
which was held in New York City earlier this week. If you’re in medical device
sales, I hope you found this article especially useful and informative. Please
leave a comment below and share this with your colleagues and friends.













I love learning about new medical technology. Truly some amazing new inventions happening out there.
ReplyDelete