| The Yes Album (Photo credit: Wikipedia) |
One of my favorites is the title of a song by the award-winning, decades-old prog rock group, Yes, from it's third release, "The Yes Album." The clever song title is appropriate to life in general and, in particular, the medical technology field: Perpetual Change.
Think about it. For centuries, as long as humans have been practicing the medicinal arts, we’ve continually sought about discovering or creating new-and-improved tools to help in the diagnose, treatment, prevention and/or elimination of disease. Every quarter there are new advances in a wide range of technologies (and pharmaceuticals) that will impact our health. Again, in this ongoing series, “Fascinating Captain!” I intend to share with you recent advances in the perpetually changing field of health care technology.
Without further adieu, here’s a synopsis of some of the latest medical technology articles pertaining to a field that's perpetually changing:
FDA Medical Device Proposal Would Develop Faster Path to Market for Breakthrough Technologies
| (Photo credit: Wikipedia) |
To gain expedited approval, medical devices would need to:
∙ Treat or diagnose a life-threatening or irreversibly debilitating disease or condition
∙ Address lack of approved alternative treatment/diagnostic exits
∙ Be a breakthrough technology that provides a clinically meaningful advantage over existing technology
∙ Be a technology where its availability is in the patient’s best interest
∙ Have an acceptable data development plan that has been approved by the FDA
How Technology Will Drive the Promising Future of Medicine
| Star Trek: McCoy (Photo credit: JD Hancock) |
Just as our bathroom scales give us an instant reading of our weight, and thermometers our internal body temperature, tomorrow’s wearable devices (and even some of today’s smart phones) will be able monitor, measure and assess various aspects of our health and well-being, and can alert us when we’re going to get sick or perhaps suffer from an imminent injury (due to a weak muscle, tendon, etc.). Because we’ll have a better “window” into what’s going on aside us in real time, doctors and perhaps even our artificial devices themselves will be able to prescribe medicines, therapies or suggest lifestyle changes based on our full medical history, holistic self and genetic composition.
“Our smartphones will become a medical device akin to the ‘Star Trek’ tricorder,”states the author.
New, Smaller Pacemaker Installed for First Time in Southern California
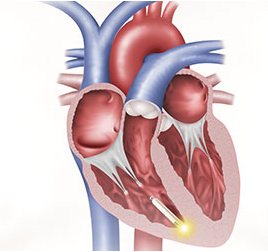 A new medical advancement in wireless pacemakers was installed for the first time in Southern California into an 83-year-old woman at San Diego's Scripps Memorial Hospital. Called the Nanostim Leadless Pacemaker, the wireless device is one-tenth the size of traditional pacemakers. According to the hospital’s officials, while still in the research phase, the Nanostim is the world’s first pacemaker that is completely implanted into a patient’s heart. The doctor who performed the surgery guided the pacemaker through a vein in the patient's groin all into her heart’s right ventricle, where, like a space station, it “undocked” to remain inside her body to perform for the next 18 years.
A new medical advancement in wireless pacemakers was installed for the first time in Southern California into an 83-year-old woman at San Diego's Scripps Memorial Hospital. Called the Nanostim Leadless Pacemaker, the wireless device is one-tenth the size of traditional pacemakers. According to the hospital’s officials, while still in the research phase, the Nanostim is the world’s first pacemaker that is completely implanted into a patient’s heart. The doctor who performed the surgery guided the pacemaker through a vein in the patient's groin all into her heart’s right ventricle, where, like a space station, it “undocked” to remain inside her body to perform for the next 18 years. Since the Nanostim is still in the research phase, potential recipients are being carefully selected. Researchers expect 600 or so procedures are needed before the FDA will make an approval decision.
Medical Devices at Risk from Cyber Attack
 Having just positively reflected upon the benefits of emerging medical technologies and devices, the medical industry at-large faces a serious issue.
Having just positively reflected upon the benefits of emerging medical technologies and devices, the medical industry at-large faces a serious issue.According to the article, “Medical devices that use a wireless connection such as pacemakers, defibrillators, monitors and insulin pumps, as well as automated drug distribution systems that are implanted in the bodies have all been considered to be at risk.” And no doubt, as more wireless devices/services become available, unless precautions are taken, they will also be vulnerable. This also means that entire health care facilities’ data storage systems are susceptible to attack. Imagine ... medical records, billing information, patients’ and hospital personnel’s key information could all be hacked, and even tampered with.
Here’s a statement from the article that’ll jump your heart into arrhythmia ... It’s by Gunter Ollmann, CTO for IOActive, who says, “The medical industry is not a thought leader of information security – it is still largely playing catch up. When I look at implanted medical technology, it is pretty scary stuff.”
Anti-fungal Coating for Medical Devices
 Like bacterial and viruses, microbial fungi are everywhere, hence the popular phrase, "fungus among us." Fungal films coat common surfaces we come into to contact with every day, and a fog of microscopic spores permeates the air (a big “Ewwwwwww!”). Thankfully, these ubiquitous microbes usually cause us no harm, but opportunistic species can take advantage of some people’s compromised immune systems, thus instigating possibly dangerous infections. For example, AIDS patients or those going through chemotherapy, inserted or implanted medical devices can serve as opportunistic vehicles for these pathogenic fungi. A team of Spanish researchers have been developing a coating for the rubber surfaces of medical devices that releases antifungal drugs when fungi are present (a big “¡Olé!”). Their strategy involves ergosterol, a cholesterol-like component of fungus cell membranes that isn’t found in mammalian membranes. While the researchers still need to prove the materials are safe and effective inside the body, future applications may include helping control fungal growth on food packaging.
Like bacterial and viruses, microbial fungi are everywhere, hence the popular phrase, "fungus among us." Fungal films coat common surfaces we come into to contact with every day, and a fog of microscopic spores permeates the air (a big “Ewwwwwww!”). Thankfully, these ubiquitous microbes usually cause us no harm, but opportunistic species can take advantage of some people’s compromised immune systems, thus instigating possibly dangerous infections. For example, AIDS patients or those going through chemotherapy, inserted or implanted medical devices can serve as opportunistic vehicles for these pathogenic fungi. A team of Spanish researchers have been developing a coating for the rubber surfaces of medical devices that releases antifungal drugs when fungi are present (a big “¡Olé!”). Their strategy involves ergosterol, a cholesterol-like component of fungus cell membranes that isn’t found in mammalian membranes. While the researchers still need to prove the materials are safe and effective inside the body, future applications may include helping control fungal growth on food packaging.Apple Adds Another Medical Device Expert

Mac medicine, anyone? According to recent reports, Apple has hired Divya Nag, a high profile medical device expert, to its ever-growing team of health-related researchers, developers, and industry strategists. Nag's former company, Stem Cell Theranostics, a drug-testing company, developed a new technology, the induced pluripotent stem cell. It turns cells — usually from a piece of skin — into embryonic-like stem cells, then uses them to create heart cells. She also founded StartX, which helps medical technology startups get on their feet.
As the article states: “Apple’s hiring of Nag is the company’s latest personnel acquisition in what has become a headline-generating pattern throughout 2014, as the company continues expanding its body of health-related experts.”
Naturally, these types of announcements continue to generate industry speculation about what new health-related technologies Apple may be developing.
Nanotechnology News: Mini-Robots May One Day Assemble Medical Devices Inside Your Body
 While “Star Trek” creator, Gene Roddenberry, was certainly ahead of his time, one idea he didn’t introduce on his original TV series: mini-robots that could perform delicate tasks inside your body, including assembling medical devices within your own bloodstream. New nanotechnology research published in “Advanced Functional Materials” recently provided some of the groundwork necessary to one day develop mini-robots that could perform delicate tasks inside your body.
While “Star Trek” creator, Gene Roddenberry, was certainly ahead of his time, one idea he didn’t introduce on his original TV series: mini-robots that could perform delicate tasks inside your body, including assembling medical devices within your own bloodstream. New nanotechnology research published in “Advanced Functional Materials” recently provided some of the groundwork necessary to one day develop mini-robots that could perform delicate tasks inside your body.Of course, having a piece of technology that floats and functions through our innards isn’t new; since 2001, the FDA approved the use of endoscopic camera pills to course through and take pix of our gastrointestinal systems.
“Nano Lego”? Nanorobots enable production in several types of items and operations. While sounding like something we’d expect to hear several years from now, already, experiments using magnetic materials have demonstrated nanorobots are capable of transporting small objects and building bridges from rods. In addition to nanotechnology’s anticipated benefits for in health care, it promises two other major advantages: as the volume of nanotechnology robotics increases, the cost will come down; and it’s anticipated the U.S.’ research and development in the science will create more jobs in the future.
New Device for Migraine Sufferers
As many as 36 million Americans suffer from migraines. These stop-you-in-the-middle-of-your-tracks headaches are a very complex disorder that involves blood vessels and nerves. It affects sufferer's heads, digestive systems (hence the nausea many experience during an attack), and causes other painful physical sensations -- even temporary blindness in some.
Now there's a wearable new device, called the Cefaly, which is designed to target a specific nerve in people's head's that's major factor in causing migraines. The Ceflay sends out signals that interrupts that key nerve's biochemical function.
Meant to be worn 20 minutes every day, a study found it reduced the number of migraines per month -- but unfortunately not their severity. Talk about oxymorons ... the most common side effect was a mild headache after the treatment session. Some mild sleepiness during the session was also reported. Last month, when it was approved in Canada, the Ceflay sold for $2,550.
It's hoped that once approved in the US, the Ceflay will help offset the cost of OTC and oftentimes expensive prescription migraine drugs, not to mention the costs of missed work days, etc. It could also be a boon to migraneurs because of not having many adverse side effects.
In this second installment of “Fascinating, Captain!” I shared some of the latest innovations and trends in medical technology featured in the news recently. If you found this article useful and informative, please leave a comment below. Also, feel free to share it with your colleagues and friends.
Rick Fromme combines entrepreneurial enthusiasm with an insider's knowledge of the medical industry to co-found MedMasters.com. Both his drive and perspective helps provide health care professionals with a superior mechanism with which to communicate, network and market their strengths. Prior to founding MedMasters.com, Rick operated a highly successful medical device distributorship. Other milestones in his 12-year career in the medical industry include a key position at a medical device start-up company that was later sold to the Ethicon Endo division of Johnson & Johnson. You may reach Rick by connecting with him on Facebook, Twitter, Google+, LinkedIn and YouTube












I want to learn more re: that new anti-migraine device!
ReplyDelete